MOVEMENT OF SUBSTANCE IN AND OUT OF CELLS
The exchange of materials is vital in the life of all organisms. Throughout life, an organ- ism continually exchange materials between itself and its surroundings, and between the cells and fluids within its body.
Importance of movement of substance in and out of cells
-
For cells to obtain nutrient.
-
Allow cells to excrete waste substances.
-
Allows cells to excrete useful substance.
-
To generate ionic gradient for muscular and nervous activity.
-
To maintain suitable pH and ionic concentration for enzyme activity.
Mechanisms for movement of molecules
Molecules move in and out of a cell through the cell membrane, which forms the boundary of each cell. The cell membrane is selectively permeable to substances, which means that it permits entry and exit of certain molecules only. The movement of molecules takes place by diffusion, osmosis, and active transport.
Types of membrane
-
Permeable membrane
It is a membrane through which molecules of both solute and solvent can pass e.g. cell wall of plants
-
Semi-permeable membrane
(or selectively permeable membrane)
It is a membrane which allows some types of substance only to pass through it. A semi-permeable membrane or partially-permeable membrane allows only solvent mole- cules but not solute molecule (dissolved solid) to pass through it.
Goat skin, pigs blad- der, cellophane, toads skin, parchment paper, visking tube
are all semi-permeable membrane. Cellophane, parchment paper and visking tube are non-ling semi-permeable membranes
Diffusion
Diffusion
is the movement of substances or molecules (either gas, liquid or solid) from a region of higher concentration to a region of lower concentration until eventually all the molecules are uniformly distributed. A dynamic equilibrium is established when the concentrations of the two regions are equal.
Diffusion occurs only when a concentration gradient exists. The concentration gradient is the difference in concentration of molecules between two regions. The steeper the concentration gradient, the faster the rate of diffusion. The rate of diffusions affected by the following factors:
Experiment
Aim:
To demonstrate diffusion in gases
Materials:
Bottle containing perfume, a room of reasonable size
Methods:
-
Open a bottle containing perfume in the corner of a room
-
Move some distance away from the bottle of perfume
-
Wait for a few minutes
Observation:
the odour of the perfume is perceived throughout the room
Conclusion:
the spread of the perfume throughout the room shows that diffusion has occurred in air.
Or
Obtain a bottle of highly scented perfume. Stand at one corner of a classroom and after closing all the doors and windows. Put two drops of the perfume on the floor and move to the opposite end of the classroom. The scent of the perfume will be perceived throughout the room which means that the particles of the perfume have moved from a region of higher concentration to a region of lower concentration
Experiment
Aim:
To demonstrate diffusion in liquids
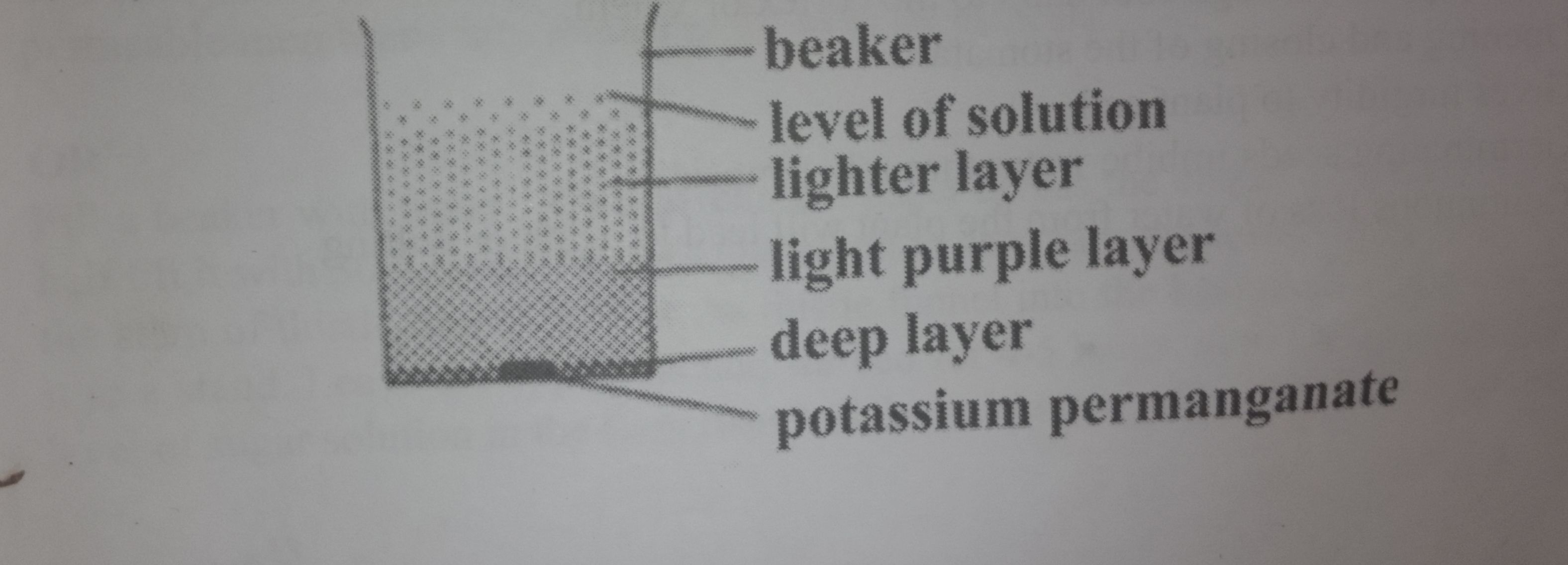
Materials:
beaker, water, crystal of potassium permanganate
Method:
-
A beaker is filled with water
-
It is left undisturbed for a few minute to get it settled
-
A crystal of potassium permanganate is carefully dropped into the water
-
The beaker is left on a bench for at least 30 minutes
Observation:
the potassium permanganate crystal will dissolve and its particles spread throughout the water turning the water violet
Conclusion:
Diffusion of potassium permanganate crystal has taken place.
Or
Half fill a beaker with water and put a grain of potassium permanganate into the water in the beaker. Leave the beaker on a flat table for about 40 minutes. After sometime the particles of the potassium permanganate spread to other parts of the water in the beaker and eventually the colour becomes the same all through the liquid. That means that the particles of potassium permanganate have diffused evenly through the water.
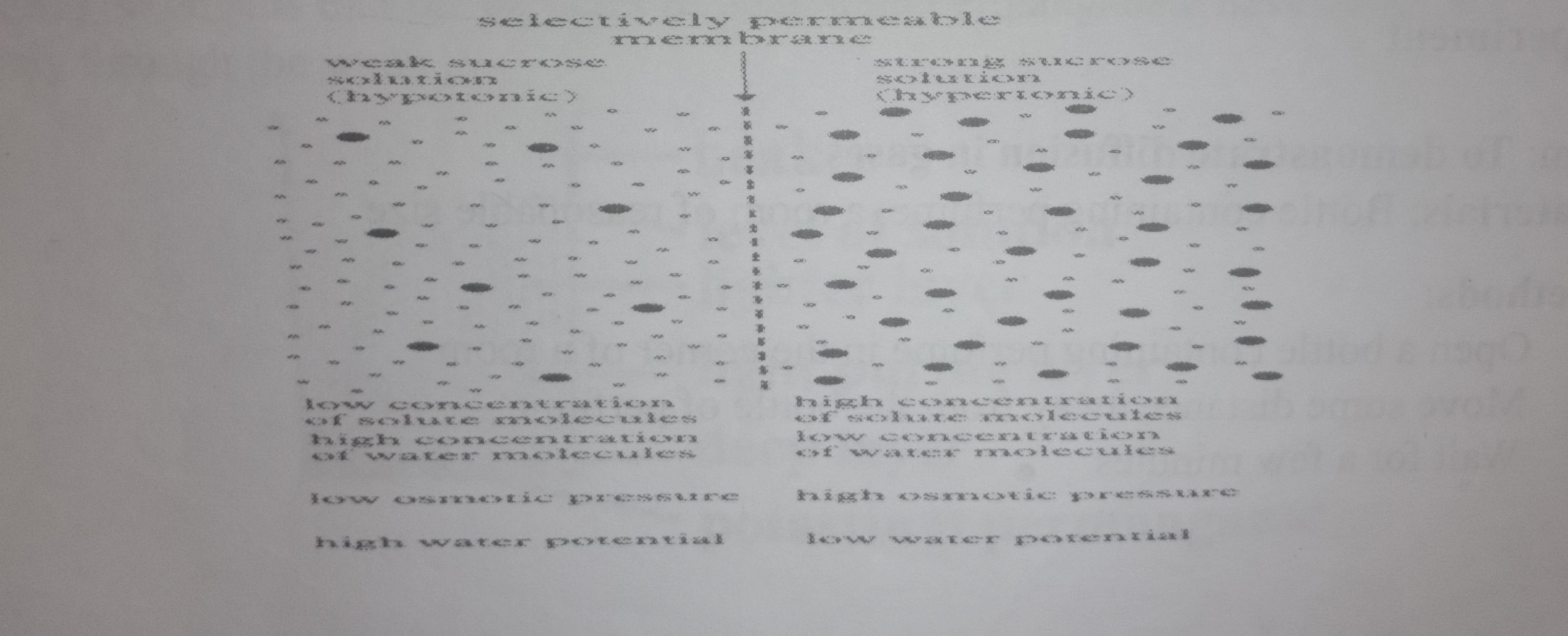
Osmosis
Osmosis is the movement of solvent molecules from a dilute solution across a semi- permeable membrane to a more concentrated solution till a dynamic equilib- rium is established when the concentrations at the two sides of the semi-permeable membrane are equal
Or
Osmosis is the movement of water molecules from a dilute solution to a concentrate solution across a semi-permeable membrane. OR
Osmosis is the movement of water molecules from a region of high water concentratio to a region of low water concentration across a semi-permeable membrane
Osmosis can be explained by the fact that less concentrated solution contains more free energy, so solvent molecules tend to diffuse to a place of lower free energy in order to equalize free energy.
Factors affecting osmosis
-
Concentration gradient - the greater the difference in concentration between the two solution, the faster the rate of osmosis
-
Semi-permeable membrane or permeability of the membrane
-
Temperature- the higher the temperature, the faster the rate of osmosis
Biological importance of osmosis/ examples of osmosis
-
In humans
-
Reabsorption of water in the tubules of the nephrons of the kidney
-
Reabsorption of water from the large intestines into the blood stream
-
Haemolysis and crenation of red blood cells. Saltwater from the ocean is hypertonic to the cells of the human body; the drinking of ocean water dehydrates body tissues instead of quenching thirst.
-
In plants
-
Absorption of water from soil into the vacuole of root hair
-
Water uptake through root hairs to the cortex or xylem
-
Opening and closing of the stomata
-
Gives turgidity to plant cell
-
Germinating seeds imbibe water through osmosis.
-
Continuous loss of water from the plant will lead to death or wilting
Experiment:
Aim:
To demonstrate osmosis in non-living material.
Materials:
Visking tube, beaker, sugar solution, thistle funnel, clamp and stand, water.
Osmosis using non-living material semi-permeable membrane
Methods:
-
The mouth of the thistle funnel is tied with visking tube.
-
The thistle funnel is filled half-way the stem with a strong sugar solution (30% sugar solution)
-
The initial level of the sugar solution is marked
-
The thistle funnel is placed into a beaker containing water with the covered end inside the water
-
A control experiment is set up in which water is used to fill both the beaker and the thistle funnel
-
The level of the sugar solution is observed at regular intervals for about six hours